99.7% of people stayed HIV-negative while on
DESCOVY for PrEP® in a 2-year study
The DISCOVER trial recruited 5,000+ people. All study participants were either men or transgender women who have sex with men with diverse backgrounds and sexual practices who didn't always use condoms. Some participants had sexually transmitted infections.
The results were analyzed when everyone had been in the study for at least 2 years (~96 weeks), at that time:


Common side effects in people taking DESCOVY for PrEP are diarrhea, nausea, headache, fatigue, and stomach pain. Tell your healthcare provider if you have any side effects that bother you or do not go away. Learn more about the possible side effects here.
In the study, PrEP medicines worked best when taken every day.
* 1 of these 8 people was suspected to have HIV before the study began.
† TRUVADA (emtricitabine 200 mg/tenofovir disoproxil fumarate 300 mg) tablets.
‡ 4 of these 15 people were suspected to have HIV before the study began.
Ask your healthcare provider if DESCOVY for PrEP is right for you.
Hear what real users think about the efficacy of DESCOVY.
Hear what real users think about the efficacy of DESCOVY.
TEXT ON-SCREEN:
Descovy®
emtricitabine 200mg/
tenofovir alafenamide 25mg tablets
By prescription only.
Please see Important Facts about DESCOVY®, including important warnings, at DescovyStories.com. Ask your healthcare provider if DESCOVY is right for you.
TEXT ON-SCREEN:
HEAR WHAT REAL USERS HAVE TO SAY ABOUT DESCOVY®
Real users compensated by Gilead.
Please see Important Facts about DESCOVY®, including important warnings, at DescovyStories.com. Ask your healthcare provider if DESCOVY is right for you.
ANNOUNCER 1:
Hear what real users have to say about Descovy.
TEXT ON-SCREEN:
DESCOVY for PrEP® (pre-exposure prophylaxis) helps protect against getting HIV through sex. It’s not for everyone. DESCOVY for PrEP is not for people assigned female at birth. Talk to your healthcare provider to find out if it’s right for you.
ANNOUNCER 2:
DESCOVY for PrEP is a once-daily prescription medicine that helps protect against HIV. It’s not for everyone. DESCOVY for PrEP is not for people assigned female at birth. Talk to your healthcare provider to find out if DESCOVY, the smallest PrEP pill available, is right for you.
TEXT ON-SCREEN:
WHAT DO YOU THINK ABOUT DESCOVY for PrEP® BEING 99.7% EFFECTIVE* IN PREVENTING HIV IN A CLINICAL TRIAL?
*Similarly, 99.4% stayed HIV-negative with TRUVADA®. Results from a 2-year study. Learn more at DescovyResults.com.
ANNOUNCER 1:
What do you think about DESCOVY for PrEP being 99.7% effective in preventing HIV in a clinical trial?
TEXT ON-SCREEN:
DESCOVY® should be taken once daily as prescribed.
KEVIN:
I think knowing how effective it could be really helped with helping me to
TEXT ON-SCREEN:
KEVIN
DESCOVY® user since 2020
LAMONT
DESCOVY® user since 2020
DESCOVY® should be taken once daily as prescribed.
KEVIN:
…decide to speak to my healthcare provider about it just to get the whole process started.
LAMONT: Knowing how effective it can be, it really makes me feel like I’m able to help protect myself.
TEXT ON-SCREEN:
SERGIO
DESCOVY® user since 2020
EVERETT
DESCOVY® user since 2019
Similarly, 99.4% stayed HIV-negative with TRUVADA®. Results from a 2-year study. Learn more at DescovyResults.com.
EVERETT:
I did know that DESCOVY for PREP was over 99% effective, and that was one of the reasons why I talked to my healthcare provider about it.
TEXT ON-SCREEN:
Descovy®
DESCOVY® doesn’t prevent other sexually transmitted infections, so use safer sex practices.
ANNOUNCER 2:
DESCOVY doesn’t prevent other sexually transmitted infections, so use safer sex practices.
TEXT ON-SCREEN:
You must test HIV-negative before and at least every 3 months while taking DESCOVY®.
ANNOUNCER 2:
You must test HIV-negative before and at least every 3 months while taking DESCOVY.
TEXT ON-SCREEN:
Tell your healthcare provider right away if you think you were exposed to HIV or have flu-like symptoms. They may want to test you for HIV.
ANNOUNCER 2:
Tell your healthcare provider right away if you think you were exposed to HIV or have flu-like symptoms. They may want to test you for HIV.
TEXT ON-SCREEN:
Serious side effects can occur, including kidney problems and kidney failure.
ANNOUNCER 2:
Serious side effects can occur, including kidney problems and kidney failure.
TEXT ON-SCREEN:
Rare, life-threatening side effects include a build-up of lactic acid and liver problems. The most common side effect was diarrhea.
ANNOUNCER 2:
Rare, life-threatening side effects include a buildup of lactic acid and liver problems. The most common side effect was diarrhea.
TEXT ON-SCREEN:
Tell your healthcare provider about all the medicines and supplements you take, or if you have kidney or liver problems.
ANNOUNCER 2:
Tell your healthcare provider about all the medicines and supplements you take, or if you have kidney or liver problems.
TEXT ON-SCREEN:
If you have hepatitis B, don’t stop taking DESCOVY® without talking to your healthcare provider, as your hepatitis B may get worse.
ANNOUNCER 2:
If you have hepatitis B, don’t stop taking DESCOVY without talking to your healthcare provider, as your hepatitis B may get worse.
TEXT ON-SCREEN:
Descovy®
emtricitabine 200mg/
tenofovir alafenamide 25mg tablets
for (PrEP) pre-exposure prophylaxis
Ask your healthcare provider if DESCOVY® is right for you.
Please see Important Facts, including important warnings, at DESCOVY.com.
DESCOVY for PrEP, the DESCOVY for PrEP Logo, the DESCOVY Blue Pill Design, DESCOVY, the DESCOVY Logo, GILEAD, and the GILEAD Logo are trademarks of Gilead Sciences, Inc., or its related companies.
[Gilead logo] ©2025 Gilead Sciences, Inc. All rights reserved. US-DVYC-0230 02/25
ANNOUNCER 2:
Ask your healthcare provider if DESCOVY for PrEP is right for you.
DESCOVY had less impact on bone health than TRUVADA
Bone mineral density, or BMD, is a good gauge of bone health. And for most people, our bones actually continue to grow into our 30s.
For people taking DESCOVY in the DISCOVER trial, their BMD was less impacted than those taking TRUVADA (at about 2 years or ~96 weeks):
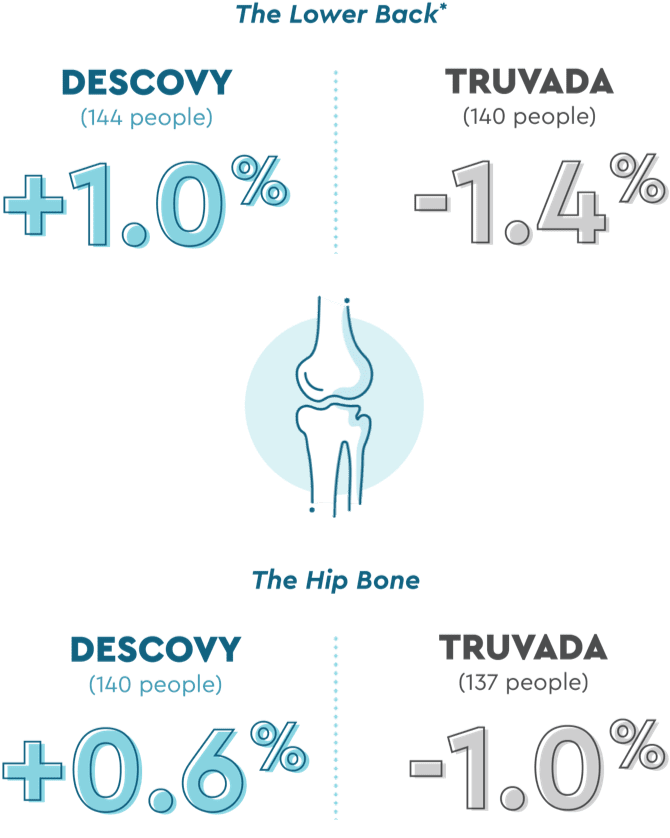
BMD was measured in study participants when they entered the study and again at 2 years from the start of the study.
The long-term significance of these changes in BMD is not known.
*Also known as the lumbar spine. Your lumbar spine consists of the five bones (vertebra) in your lower back.

DESCOVY had less impact on kidney function lab tests than TRUVADA
Kidneys help keep you healthy by removing waste from your blood, that’s why it’s important to take care of them. DESCOVY for PrEP works just as well at helping prevent HIV as TRUVADA, with less impact on your kidneys.
DESCOVY may cause new or worsening kidney problems, including kidney failure. Your healthcare provider should do blood and urine tests to check your kidneys before you start and while taking DESCOVY. Your healthcare provider may tell you to stop taking DESCOVY if you develop new or worse kidney problems.
In the DISCOVER trial, those taking DESCOVY had less impact on certain kidney function lab tests than those taking TRUVADA (at about 2 years or ~96 weeks):
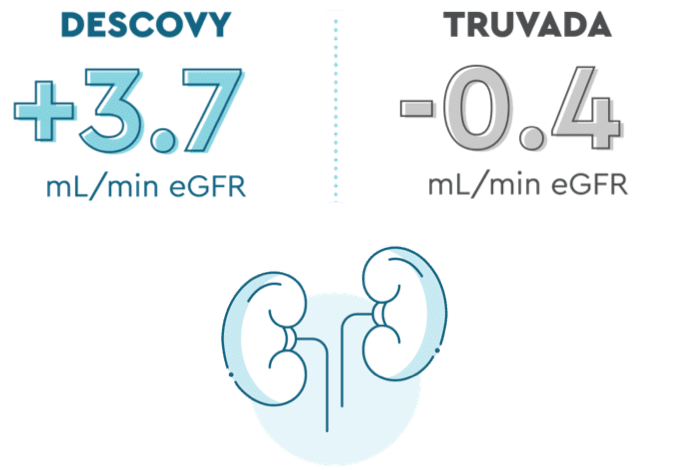
eGFR is one of the measurements that your healthcare provider may use to see how your kidneys are functioning. eGFR was measured in study participants when they entered the study and again at 2 years from the start of the study.
The long-term significance of these changes on the frequency of kidney side effects is not known.
Discover more about DESCOVY for PrEP:

Hear what inspires real users to share their experiences with DESCOVY for PrEP.

Learn about the possible side effects of DESCOVY for PrEP.

See how DESCOVY for PrEP could cost as little as $0. Restrictions apply.
DESCOVY for PrEP (pre-exposure prophylaxis) is a once-daily prescription medicine for adults and adolescents at risk of HIV. It helps lower the chances of getting HIV through sex.
DESCOVY for PrEP is not for everyone. It is not for use in people assigned female at birth who are at risk of getting HIV from vaginal sex, because its effectiveness has not been studied. You must be HIV-negative before and while taking DESCOVY for PrEP.
DESCOVY for PrEP is not for everyone:
- It is not for use in people assigned female at birth who are at risk of getting HIV from vaginal sex, because its effectiveness has not been studied.
- You must be HIV-negative before and while taking DESCOVY for PrEP.
Talk to a healthcare provider to see if DESCOVY for PrEP may be an option for you.
Important Safety Information
What is the most important information I should know about DESCOVY for PrEP?
Before and while taking DESCOVY for PrEP:
Tap for Important Safety Information, including important warnings on the risk of drug resistance if you become HIV-positive and only take DESCOVY, and worsening of hepatitis B infection.
- You must be HIV-negative before you start and while taking DESCOVY for PrEP. You must get tested for HIV-1 immediately before and at least every 3 months while taking DESCOVY. If you think you were exposed to HIV-1, tell your healthcare provider right away. They may want to do more tests to confirm that you are still HIV-negative.
- Many HIV-1 tests can miss HIV-1 infection in a person who has recently become infected. Tell your healthcare provider if you had a flu-like illness within the last month before starting or while taking DESCOVY. Symptoms of new HIV-1 infection include tiredness, fever, joint or muscle aches, headache, sore throat, vomiting, diarrhea, rash, night sweats, and/or enlarged lymph nodes in the neck or groin.
- DESCOVY by itself is not a complete treatment for HIV-1. Do not take DESCOVY for PrEP unless you are confirmed to be HIV-1 negative.
- DESCOVY does not prevent other sexually transmitted infections (STIs). Practice safer sex by using a latex or polyurethane condom to reduce the risk of getting STIs.
- To further help reduce your risk of getting HIV-1:
- Do not miss any doses of DESCOVY. Missing doses may increase your risk of getting HIV-1.
- Know your HIV status and the HIV status of your partners. If your partner is living with HIV, your risk of getting HIV is lower if your partner consistently takes HIV treatment every day.
- Get tested for other STIs. Some STIs make it easier for HIV-1 to infect you.
- Talk to your healthcare provider about all the ways to help reduce HIV risk.
DESCOVY can cause serious side effects:
- Worsening of hepatitis B (HBV) infection. Your healthcare provider will test you for HBV. If you have HBV and stop taking DESCOVY, your HBV may suddenly get worse. Do not stop taking DESCOVY without first talking to your healthcare provider, as they will need to check your health or give you HBV medicine.
Who should not take DESCOVY for PrEP?
Do not take DESCOVY for PrEP if you:
- Already have HIV-1 or if you do not know your HIV-1 status. If you have HIV-1, you need to take other medicines with DESCOVY to treat HIV-1. If you have HIV-1 and take only DESCOVY, your HIV-1 may become harder to treat now and in the future.
What are the other possible side effects of DESCOVY for PrEP?
Serious side effects of DESCOVY may also include:
- Kidney problems, including kidney failure. Your healthcare provider should do blood and urine tests to check your kidneys before and during treatment with DESCOVY. If you develop kidney problems, your healthcare provider may tell you to stop taking DESCOVY.
- Too much lactic acid in your blood (lactic acidosis), which is a serious but rare medical emergency that can lead to death. Tell your healthcare provider right away if you get these symptoms: weakness or being more tired than usual, unusual muscle pain, being short of breath or fast breathing, stomach pain with nausea and vomiting, cold or blue hands and feet, feel dizzy or lightheaded, or a fast or abnormal heartbeat.
- Severe liver problems, which in rare cases can lead to death. Tell your healthcare provider right away if you get these symptoms: skin or the white part of your eyes turns yellow, dark "tea-colored" urine, light-colored stools, loss of appetite for several days or longer, nausea, or stomach-area pain.
Common side effects in people taking DESCOVY for PrEP are diarrhea, nausea, headache, fatigue, and stomach pain. Tell your healthcare provider if you have any side effects that bother you or do not go away.
What should I tell my healthcare provider before taking DESCOVY for PrEP?
- All your health problems. Be sure to tell your healthcare provider if you have or have had any kidney or liver problems, including hepatitis.
- All the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. DESCOVY may interact with other medicines. Keep a list of all your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see Important Facts about DESCOVY for PrEP, including important warnings.
